MADRID, 13 Mar. (EUROPA PRESS) -
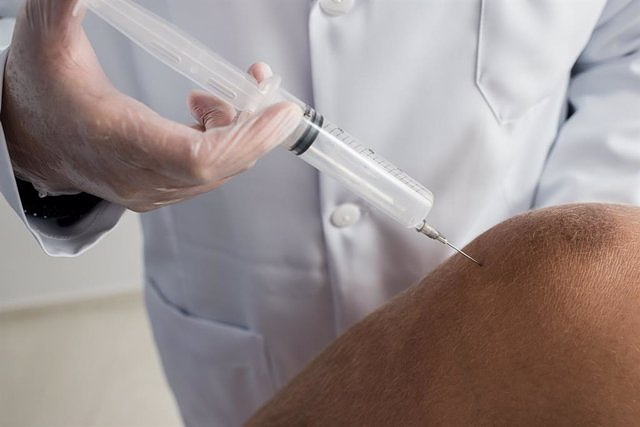
The European Center for Disease Control and Prevention (ECDC) has alerted this Monday of an outbreak of at least 14 cases of botulism (12 in Germany, one in Austria and one in Switzerland) in European citizens who traveled to Istanbul (Turkey) between February 22 and 25 to receive gastric injections of botulinum neurotoxin, which is administered to aid weight loss.
Intragastric botulinum toxin injection in animals and humans is sometimes used as a treatment for obesity. Its effect would include the delay of gastric emptying and the induction of early satiety, with the consequent decrease in intake and weight loss. However, it carries more risks than other weight loss operations.
According to the ECDC communicable disease bulletin, all the cases are middle-aged adults and ten of them (8 from Germany and those affected in Austria and Switzerland) received treatment at the same clinic.
In the case of patients from Germany, there are as many mild as severe cases. At the moment, there have been several hospitalizations and "several" have required admission to Intensive Care Units (ICU).
According to the information currently being handled by the European body, for the moment there are no indications that the treatment was organized by a commercial medical travel company. Instead, information received from patient interviews indicates that a WhatsApp group was used to contact the hospital.
Three of the cases in Germany recall the name of the product used in their operations, and in all of them it matched, so it seems clear that the outbreak is localized to that particular Turkish clinic.
The case of Austria is a woman between the ages of 25 and 44 who received this treatment on February 22. According to this patient, her injection was "self-administered" at the clinic itself. The woman was hospitalized with symptoms of botulism (ptosis, dysphagia, dyspnea, neck weakness, generalized muscle weakness).
Meanwhile, the Swiss case remains suspicious and is not yet confirmed. She is also a woman, in this case between 45 and 64 years old, who also received the injection on February 22 at the suspected clinic.
European authorities fear that more cases will come to light, "taking into account the possible variation in the clinical presentation of botulinum disease." "New cases may occur, especially among people traveling to Turkey to undergo medical treatments involving intragastric injection of botulinum neurotoxin," they note.
The ECDC advises people who have traveled to Istanbul to undergo this treatment and present symptoms compatible with botulism (weakness, difficulty breathing and/or swallowing) to see a doctor "as soon as possible".
This is not the first time that the ECDC has investigated similar cases of botulism. In 2019, France reported a suspected case of botulism in a 25-44-year-old woman after an intragastric injection of botulinum neurotoxin in Egypt for weight loss. Later, she had to be hospitalized upon her return to France.
In addition, a World Health Organization (WHO) alert on medical products from August 2022 reported five counterfeit batches of botulinum neurotoxin in five countries: Jordan (May 2022), Turkey (May 2022), Kuwait (June 2022), 2022), UK (June 2022) and Poland (July 2022). However, ECDC "does not know if these batches have been used for treatment in the cases reported so far."
Outbreaks of iatrogenic botulism, sometimes related to counterfeit or unauthorized botulinum neurotoxin, have also been reported in the past following different clinical procedures, for example in Egypt and Turkey.
Botulism is a serious neuroparalytic disease caused mainly by botulinum toxin, a product of the 'Clostridium botulinum' bacterium. The disease occurs naturally in four different forms: foodborne, intestinal, infant, and wound botulism.
There are two other forms of botulism that do not occur naturally: inhalation and iatrogenic, which is the newer form of man-made botulism, mostly in medical treatments with few guarantees. Thus, poisoning can occur as an adverse event after the administration of botulinum neurotoxin for therapeutic or cosmetic reasons.
Although considered rare, people receiving botulinum neurotoxin injections for cosmetic purposes (for example, for facial wrinkles) or therapeutic treatments (for example, for the treatment of muscle spasticity) may develop iatrogenic botulism if injected with a excessive dose.
The symptoms of iatrogenic botulism are characterized by weakness and difficulty swallowing. Toxicities after cosmetic treatment include ophthalmologic and oropharyngeal symptoms (blurred vision, drooping eyelid, difficulty swallowing, and dry mouth), while toxicities after therapeutic treatment are related to shortness of breath and weakness.
The symptoms of botulism can be very severe and require intensive care treatment, as well as administration of an antitoxin. Even when these treatments are available, full recovery often takes weeks or months, as the ECDC recalls. In the case of foodborne botulism, between 5 and 10 percent of cases are fatal.

 Exploring Cardano: Inner Workings and Advantages of this Cryptocurrency
Exploring Cardano: Inner Workings and Advantages of this Cryptocurrency Seville.- Economy.- Innova.- STSA inaugurates its new painting and sealing hangar in San Pablo, for 18 million
Seville.- Economy.- Innova.- STSA inaugurates its new painting and sealing hangar in San Pablo, for 18 million Innova.- More than 300 volunteers join the Andalucía Compromiso Digital network in one month to facilitate access to ICT
Innova.- More than 300 volunteers join the Andalucía Compromiso Digital network in one month to facilitate access to ICT Innova.-AMP.- Ayesa acquires 51% of Sadiel, which will create new technological engineering products and expand markets
Innova.-AMP.- Ayesa acquires 51% of Sadiel, which will create new technological engineering products and expand markets Hamas views Israel's latest deal proposal in "positive spirit"
Hamas views Israel's latest deal proposal in "positive spirit" The Ibex 35 rises 0.22% mid-session driven by Aena (4.66) and Sabadell (4.57%)
The Ibex 35 rises 0.22% mid-session driven by Aena (4.66) and Sabadell (4.57%) STATEMENT: Selena Romero and Roberto Pérez winners of the 22nd Nacho Juncosa Memorial - International under-16 tennis tournament
STATEMENT: Selena Romero and Roberto Pérez winners of the 22nd Nacho Juncosa Memorial - International under-16 tennis tournament STATEMENT: DH2 Energy is the winner in the first European renewable hydrogen auction
STATEMENT: DH2 Energy is the winner in the first European renewable hydrogen auction How Blockchain in being used to shape the future
How Blockchain in being used to shape the future Not just BTC and ETH: Here Are Some More Interesting Coins Worth Focusing on
Not just BTC and ETH: Here Are Some More Interesting Coins Worth Focusing on UPV students design an app that helps improve the ventilation of homes in the face of high temperatures
UPV students design an app that helps improve the ventilation of homes in the face of high temperatures Ivace and promotes a less invasive device for the early detection of prostate cancer
Ivace and promotes a less invasive device for the early detection of prostate cancer Valencia unanimously approves the ordinance to allocate spaces to test innovative initiatives
Valencia unanimously approves the ordinance to allocate spaces to test innovative initiatives UPV researchers promote a paid master's degree as a "talent factory" in integrated photonics
UPV researchers promote a paid master's degree as a "talent factory" in integrated photonics A million people demonstrate in France against Macron's pension reform
A million people demonstrate in France against Macron's pension reform Russia launches several missiles against "critical infrastructure" in the city of Zaporizhia
Russia launches several missiles against "critical infrastructure" in the city of Zaporizhia A "procession" remembers the dead of the Calabria shipwreck as bodies continue to wash up on the shore
A "procession" remembers the dead of the Calabria shipwreck as bodies continue to wash up on the shore Prison sentences handed down for three prominent Hong Kong pro-democracy activists
Prison sentences handed down for three prominent Hong Kong pro-democracy activists ETH continues to leave trading platforms, Ethereum balance on exchanges lowest in 3 years
ETH continues to leave trading platforms, Ethereum balance on exchanges lowest in 3 years Investors invest $450 million in Consensys, Ethereum incubator now valued at $7 billion
Investors invest $450 million in Consensys, Ethereum incubator now valued at $7 billion Alchemy Integrates Ethereum L2 Product Starknet to Enhance Web3 Scalability at a Price 100x Lower Than L1 Fees
Alchemy Integrates Ethereum L2 Product Starknet to Enhance Web3 Scalability at a Price 100x Lower Than L1 Fees Mining Report: Bitcoin's Electricity Consumption Declines by 25% in Q1 2022
Mining Report: Bitcoin's Electricity Consumption Declines by 25% in Q1 2022 Oil-to-Bitcoin Mining Firm Crusoe Energy Systems Raised $505 Million
Oil-to-Bitcoin Mining Firm Crusoe Energy Systems Raised $505 Million Microbt reveals the latest Bitcoin mining rigs -- Machines produce up to 126 TH/s with custom 5nm chip design
Microbt reveals the latest Bitcoin mining rigs -- Machines produce up to 126 TH/s with custom 5nm chip design Bitcoin's Mining Difficulty Hits a Lifetime High, With More Than 90% of BTC Supply Issued
Bitcoin's Mining Difficulty Hits a Lifetime High, With More Than 90% of BTC Supply Issued The Biggest Movers are Near, EOS, and RUNE during Friday's Selloff
The Biggest Movers are Near, EOS, and RUNE during Friday's Selloff Global Markets Spooked by a Hawkish Fed and Covid, Stocks and Crypto Gain After Musk Buys Twitter
Global Markets Spooked by a Hawkish Fed and Covid, Stocks and Crypto Gain After Musk Buys Twitter Bitso to offset carbon emissions from the Trading Platform's ERC20, ETH, and BTC Transactions
Bitso to offset carbon emissions from the Trading Platform's ERC20, ETH, and BTC Transactions Draftkings Announces 2022 College Hoops NFT Selection for March Madness
Draftkings Announces 2022 College Hoops NFT Selection for March Madness