An OG Bitcoin investor recently made a significant move by transferring 2,000 BTC, valued at $178 million, to Coinbase. This investor had been holding onto their Bitcoin stash since 2010, when the price of Bitcoin was a mere $0.06 per coin and the market cap was around $250,000. Back then, the daily trading volume rarely exceeded $60,000.
The transfer of such a large amount of Bitcoin to an exchange like Coinbase typically indicates that the BTC will be liquidated. This activity is part of a trend where dormant Bitcoin wallets are becoming active again due to the recent surge in prices across the market following the U.S. election win of Donald Trump. Glassnode data shows an increase in the number of wallets that have been inactive for over five years, reaching a two-month high.
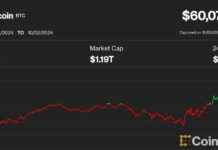
Bitcoin is currently trading at $88,532, having cooled off from its recent rally that saw it reach a record high of $93,214. This isn’t the first time that older Bitcoin wallets have started to become active as the price of Bitcoin hits new all-time highs. There have been instances this year where millions of BTC from the early days of Bitcoin, known as the “Satoshi Era” (late 2009 to 2011), were moved from dormant wallets. It’s hard to determine if these Bitcoins were sold, but given the massive profits that could be made at current prices, it’s a possibility.
The resurgence of older wallets that have been holding Bitcoin since its early days could continue as users look to capitalize on the current price levels. However, it’s worth noting that between 3-4 million BTC has been deemed “lost forever” due to irretrievable private keys, according to Chainalysis. This means that some of these OG wallets may never be able to cash out their holdings.
The trend of these older wallets becoming active could potentially limit any further increase in Bitcoin’s price, even though some traders remain optimistic that Bitcoin could reach $100,000 by the end of the year, a key psychological resistance level.
Overall, the movement of such a large amount of Bitcoin by an OG investor highlights the potential for significant profits in the cryptocurrency market, but also serves as a reminder of the risks and challenges associated with holding digital assets for an extended period of time. As the cryptocurrency industry continues to evolve and mature, it will be interesting to see how these OG investors navigate the increasingly complex landscape of digital assets and blockchain technology.